MBI Videos
Richard White
-
 Richard White
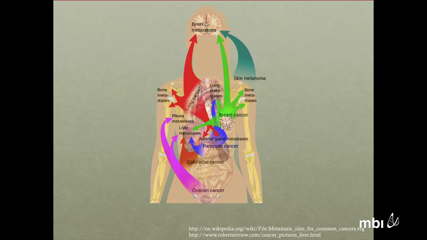
Richard WhiteMetastatic disease is the defining feature of advanced malignancy, yet the mechanisms by which it occurs and affects host physiology are poorly understood. Comprehensive genomic studies of human metastatic cancers has revealed striking heterogeneity both within primary tumors, but also between different metastases from the same patient. For these reasons, models which capture this heterogeneity will be necessary to design effective strategies to abrogate the metastatic phenotype. The zebrafish has recently emerged as a genetic model system in which to study cancer because of several key strengths: 1) small size allows for study of vast numbers of animals, 2) optical transparency facilitates in vivo imaging of even single cancer cells, and 3) amenability to unbiased genetic and small molecule screens. My laboratory has developed a zebrafish model of melanoma in which the BRAFV600E allele is expressed in the mitf+ melanocyte lineage. These animals develop stereotyped, 100% penetrant melanomas when crossed to a p53-/- mutant. Using highly sensitive fluorescent detection and automated imaging algorithms, we have defined metastatic capacity of these tumors, and demonstrate a metastasis initiating cell frequency of 1/250,000 cells. These cells show preferential metastases to locations such as skin, bone marrow, and eye. Using this as a platform, we are now defining the genomic characteristics of the metastatic clones, and designing unbiased screens to find genetic or epigenetic pathways that modify metastatic progression. The zebrafish offers a highly scalable in vivo system for interrogating the dynamics of metastasis over both time and space at a resolution unavailable in other model systems.